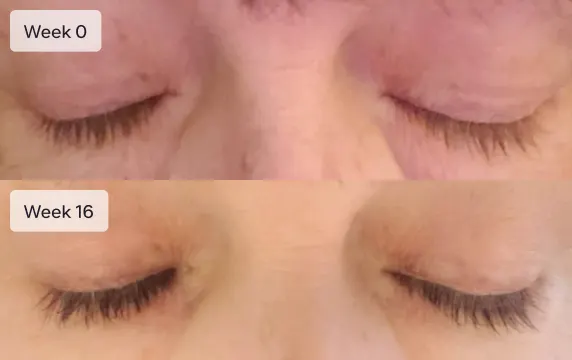
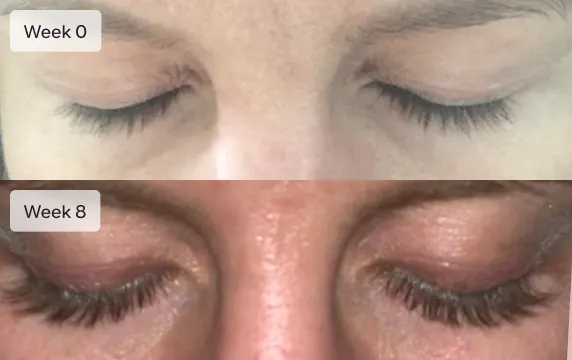
Get Longer Lashes
Latisse® (bimatoprost ophthalmic solution)
181 reviews

25%
longer
106%
fuller
18%
darker
Free, discreet shipping
Free, unlimited follow-ups
Results typically seen in 16 weeks
Pause anytime


Why Latisse?
It's the first and only FDA-approved eyelash growth serum that's clinically proven to make eyelashes longer, thicker and darker.
Meet other lash lovers who use Latisse
"My results have been so much better than I dared hope."
"I have lighter hair and I was so impressed with how thick and dark my lashes grew in."
See Latisse love in action
These testimonials were submitted by verified Ro members who were prescribed Latisse. The members were compensated and received their treatment at no cost for offering their photos and testimonials
“I haven’t been this happy with my lashes since they were fake”
Kaleigh, Ro Derm member
"This has become a regular part of my nightly skincare routine"
Khloe, Ro Derm member
“What I really love about Latisse is that it’s super convenient”
Benedicta, Ro Derm member
“My lashes have never naturally looked like this ever in my life”
Sierra, Ro Derm member
Latisse
Rx Requires prescription through online visit with a US-licensed healthcare practitioner


Latisse 5 mL Rx
Free online visit
3-month supply
Free shipping
What should you know before taking Latisse.
Real members, real reviews
Your goals are our goals, and we love hearing about how things are going.
It works for me it will work for you too!
I went from barely visible eyelashes to model length in only 8 weeks. My family and friends all noticed the dramatic difference in my lashes
Great stuff , great customer service too
Be patient in the beginning, after 6 weeks it’s amazing , love not wearing fake eyelashes as they ruin your natural ones 🥰
Great Service!
I have used Latisse for over a year. Ro provides great service and the ability to hold deliveries if you do not need a refill. Great product with no side effects for me.
Amazing product
Amazing product from an amazing and helpful site.
Love it!
Love it! Lashes are definitely longer and a bit thicker.
Getting started
How it works
Free visit
Complete an online visit and connect with a US‑licensed healthcare provider.
Free shipping
If prescribed, your treatment will be sent with free shipping.
Free and unlimited follow-ups
Send your provider a message any time to discuss updating your treatment, side effects, or any other questions or concerns.
Patient Reviews
4.86 (181 reviews)
6/27/2023
Alsina
Best Lashes
Excellent serum! Great results
6/27/2023
Alsina
6/20/2023
Ellinor
Love it
My eyelashes look great
6/20/2023
Ellinor
6/17/2023
Robin
Awesome Product
I love how Latisse took my already longish lashes to an unbelievable length. Great product that's worth the cost.
6/17/2023
Robin


Why Ro
Ro provides dermatologist-backed offerings from the comfort of your own phone. With Ro, you’ll get the support you need to live a healthy and confident life.
Important safety information
What you should know before taking Latisse.
FAQ
Have a question not listed here? Reach out to our care team and we’ll be happy to help. To connect with a care representative, email us at [email protected].
Latisse (bimatoprost solution 0.03%) is an FDA-approved treatment to grow eyelashes for people with inadequate or not enough lashes. Latisse is clinically-proven to make eyelashes longer, thicker, and darker.
Latisse is not appropriate for everyone. To learn more about who should not use Latisse and the side effects that it may cause, please see the FAQ below regarding side effects and the important safety information for Latisse.
Latisse should be used at night after washing your face, removing makeup and contacts if you wear them. Apply one drop of Latisse to an applicator. Brush the applicator along the skin of the upper eyelid, where the skin meets the eyelashes. Blot away any excess liquid to avoid liquid running onto your cheek. Use one applicator for each eye and throw away after use. Only apply at the base of the upper lashes. If you have any questions about your medication, once prescribed, please contact your provider.
Common side effects include itchy and red eyes. Hair may grow outside the treatment area, especially if the medication is applied directly to the eye or the lower eyelid. Latisse may cause brown darkening of the colored part (iris) of your eye which is likely permanent and occurred in less than 4% of patients in the Latisse clinical trial. Latisse may also cause eyelid skin darkening which may be reversible. If Latisse is discontinued, lashes gradually return to the previous appearance. For a complete list of interactions and potential side effects, please read the important safety information.
Yes, our prescriptions are brand-name Latisse (bimatoprost solution 0.03%) and purchased directly from the manufacturer, Allergan.
Eyelashes will not grow overnight. It will take 16 weeks (4 months) to see the full effect of Latisse. Most patients report an increase in eyelash length at 4 weeks. Increased darkness and fullness are typically seen at week 8. Continuing with the treatment past week 8 usually results in longer, fuller, thicker, and darker eyelashes.